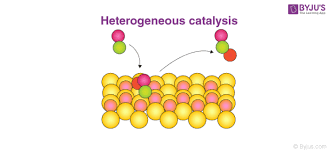
First, let us understand what is meant by Adsorption and what is meant by Heterogeneous catalysis. This type of Catalysis is where the catalyst will occupy a phase that is not the same as the reactants and products. They may be in a physical state – i.e. Solid, Liquid, and Gas and also extend to immiscible fluids.
Adsorption means the process when the atoms, molecules from a gas, liquid, or solid will get themselves stuck to the surface which is like adhesion of the atoms and molecules. The process will thus make a film on the surface of the adsorbent by the adsorbate. This is entirely different from Absorption which is the process of movement of fluids (the absorbate) when it is dissolved by a liquid and solid which is the absorbent. Adsorption is a basic surface process and if we compare it to the process of absorption it is the whole volume process. The process is called sorption because it means both processes while the process called desorption is the opposite to it.
Old adsorption theory means that the reactants when in the Gas state or dissolved state in any solution get adsorbed on the surface where a catalyst is available in the solid-state. When the process is over the catalyst is regenerated.
The Heterogeneous catalysis thus proves in the modern adsorption theory that it is a combined state of the old theory of Adsorption along with the intern meditate theory of the compound formation and there is diffusion on the catalyst surface. 250
Here are some facts about the Adsorption theory:
- There are active sites produced when there is adhesion and that increases the rate of reaction.
- The molecules which have no affinity to the adsorbent desorb and catalyst surface becomes free for adsorption for other catalyst molecules.
- When there is contact with proteins with blood, albumin is the one that is adsorbed first. This happens because albumin is more prominent in blood than other minor proteins. The minor proteins later rearrange themselves in favor of other ones owing to surface affinity against mass law.
- The molecules which are adsorbed are resistant to washing with the same type of solvent medium when there is solution adsorption. However, the washing can change the measurement when the interaction is very low.
- The process adsorption is a result of surface energy and all the bonding requirements which may be ionic, metallic r even covalent of the atoms in the material are fulfilled by some other atoms in the material
- The atoms on the surface of the adsorbent are not surrounded by other atoms and therefore can attract the adsorbates.
- The adsorption is actually known as physisorption which is a characteristic of weak Van Der Waals forces. Or also Chemisorption which is a covalent bonding type. There is a possibility that this occurs also due to electrostatic attraction.
- This adsorption is present in the natural, physical, chemical systems and is used for industrial applications.
- The industrial application is that they act as heterogeneous catalysts. They can activate charcoal, and capture and use waste heat to provide cold water for air conditioning and other process requirements which are known as adsorption chillers.
- They are also used in synthetic resins, to increase storage capacity or carbide-derived carbons and also water purification.
- In the process of Adsorption, there is ion exchange and chromatography and these are sorption processes in which certain adsorbates are transferred from the fluid base to the surface. There are rich and insoluble particles on the vessel.
- Pharmaceutical industries have application use with adsorption theory where adsorption is used to prolong neurological exposure to species drugs or lesser-known ones.
- Adsorbents: These are characterized by being spherical pellets, rods, etc with a radius of .25 to 5 nm. They must have high resistance to abrasion and thermal stability and small pore diameters and this results in higher exposed surface area and thus have a high adsorption capacity.
- They have a pore structure and it enables fast transport of the vapors when it is a Gas.
- Industrial adsorbents fall into three categories- First is the oxygen-containing compounds which are hydrophilic and polar and include materials like Silica Gel and Zeolites. Second is the Carbon-based compounds and these are typically hydrophobic and not polar ones and include materials like graphite and activated carbons. The last one is the polymer-based compounds which are polar or non-polar.
Reference:
Doubt not is India’s most popular and number one tutoring app for students to help with their studies. This app is free for all android phones and can be used by students and teachers, especially for Mathematics and Science. This app is incredibly helpful because they use an image recognition facility and New algorithms to select correct image answers that students can access and use. This app was started as an online app in the year 2016.